Acid Base Titration Lab Report
To prepare standardize solution of sodium hydroxide and to determine the concentration of. This allows for quantitative analysis of the concentration of an unknown acid or base solution.

Acid Base Titration Lab Report Pdf
Acid-base titrations depend on the neutralization between an acid and a base when mixed in solution.

. Be sure to start the titration with the buret filled exactly to the 000 mL mark. An acid-base titration. Analysis and Calculations The experiment is a strong acid-strong base titration.
To identify the suitable indicators for. The titration proceeds until the equivalence point is reached where the number of moles. Acid-Base Titration Brianna Morrison Chemistry 111 October 11 2010 Aim.
From this experiment we can analyze concentration of base when the concentration of acid is known. The acid base indicators have the quality of responding. The acid base titration lab report is one of the most kinds of homework college students might have in their Chemistry course.
Chemistry Lab Report on standardization of acid and bases. Using the molarity of NaOH and amount of base added along with the volume of the H 2 SO 4 solution the concentration of H 2 SO 4 solution can be determined. It makes use of the naturalization reaction that occurs between acids and bases.
Trial 1 Trial 2 Trial 3 Initial volume mL 1660 060 1640 Final volume mL 3230 1640. Titration of the unknown The titration results using standardized NaOH solution are listed in Table 2. Lab Report on Acid-Base Titration Free Essay Example The chemical reaction involved in acid-base titration is known as neutralisation reaction.
DO NOT put the buret under. The buret contains a large air bubble in the tip which disappears in the course of the titration. If a magnetic stirrer is not available carefully stir the solution with a.
The following lab was an acid-base neutralizing titration. Example Calculation In. Acid-base equilibrium pH of solutions AimsObjectives.
Acid-Base Titration Pre-lab Assignment 1 Potassium hydrogen phthalate KHP is a primary standard used to determine the molarity of bases such as NaOH. The buret is contaminated with an acid solution. Most of the time several weeks is the time-frame.
A titration is a technique in which a reagent called a titrant of known concentration is used to determine the concentration of an. To standardize a solution of the base sodium hydroxide using oxalic acid dihydrate. When the acid and base react they form NaCl sodium chloride which is also known as table salt.
Rinse the burette with H 2O. CHEMISTRY 11 Acid-Base Titration FULL FORMAL LAB Toombs Titration Lab Procedure DAY ONE. The acid base indicator is an expansive organic molecule which functions in a manner that is similar to a color dye.
A small volume of the acid solution is. Check valve and seal. To determine the pH range where the indicator changes colour.
Turn on the magnetic stirrer. In addition to the sample an appropriate indicator is added to the.
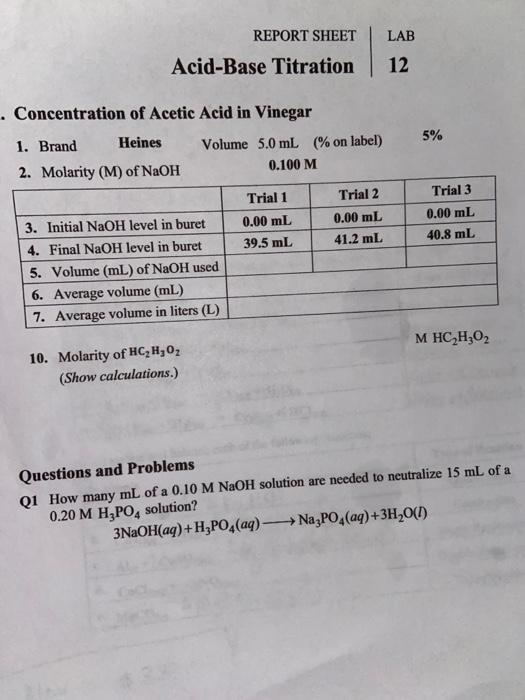
Solved Lab Report Sheet Acid Base Titration 12 Chegg Com

Solved Section Titration Lab Report Sheet Table 1 Acid Base Chegg Com


No comments for "Acid Base Titration Lab Report"
Post a Comment